TO YOUR HEALTH: New implant an alternative to blood thinners
An alternative to long-term use of blood thinners by those diagnosed with atrial fibrillation is now available at Abington Hospital-Jefferson Health.

The health system is among the first in Pennsylvania — the second in the state — to offer the newly FDA-approved Watchman Left Atrial Appendage Closure implant. The device “provides physicians with a stroke risk reduction option for patients with non-valvular atrial fibrillation,” said Dr. Richard Borge, a cardiac electrophysiologist and medical director of the Heart Rhythm Center at Abington Hospital.
Atrial fibrillation, or AF, is a heart condition in which the upper chambers of the heart beat too fast with irregular rhythm, the hospital said in a press release. The most common cardiac arrhythmia, AF affects more than 5 million Americans. “Twenty percent of all strokes occur in patients with AF, and AF-related strokes are more frequently fatal and disabling,” the release says.
What happens is the blood is not pumped efficiently, Borge said. It stagnates and can cause clots to form, usually in the left atrial appendage, which he described as a pouch that comes off the upper chamber. “Up to now the only way to combat that [clots] is blood thinners, the standard therapy for four decades,” Borge said. Some patients don’t want to take blood thinners or it’s not advisable for them to take it, he said. “Till now, there were no options.”
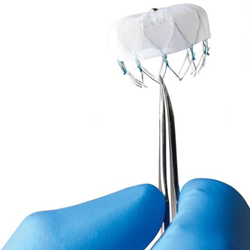
What was needed was something “not difficult to deploy, safe to deploy and effective, and deployed in a minimally invasive fashion,” he said. “All those appear to be met with the Watchman.” The Watchman is threaded through a vein in the leg and fished to the heart to the left atrium, and “when delivered, it opens up almost like an umbrella over the mouth of that appendage … the blood can’t go through that,” Borge said. “Ultimately a person’s body will heal over that. It becomes sealed.”
The manufacturer, Boston Scientific, describes the device on its website as having a self-expanding nickel-titanium frame with an attached woven plastic cap about the size of a quarter. The device is as effective as Coumadin — a brand name for warfarin — in preventing strokes, Borge said. After the implant is in place, the patient would eventually end up taking baby aspirin long-term instead of a blood thinner. The FDA approval is for non-valvular — “AF without severely damaged heart valves,” he said. It’s for patients who have not had rheumatic fever. Those who had rheumatic fever can have scarred valves and are high-risk. “It would not be appropriate for them. “AF is extremely common. Up to 20 percent of those age 80 and up [have it]. The vast majority have non-valvular AF,” he said.
Warfarin is the “standard-bearer” for treatment, Borge said. “It is very effective but is a laborious drug,” requiring regular blood tests, constant adjustment and the risk of bleeding. “Long-term warfarin medication is not well-tolerated by some patients and carries a significant risk for bleeding complications,” the release states. “Nearly half of AF patients eligible for warfarin are currently untreated due to tolerance and adherence issues.”
“Watchman is equally as effective as Coumadin without the bleeding baggage,” Borge said.
“It’s not appropriate for everyone,” he said, noting there is always some risk with an invasive procedure.
There is a small risk with any surgical procedure, he said, but once it’s in place the risk of bleeding from long-term use of a blood thinner is gone. Terming Watchman “a useful alternative for those who do not tolerate blood thinners,” Borge said an estimated 10 percent to 20 percent of AF patients would be good candidates. Since it’s a relatively new procedure and new device, it is being recommended in a conservative fashion, with a limited release, he said. “Abington has a very busy, active electrocardiology department — high volume, good track record,” Borge said. “We were fortunate to be picked.”
Borge and his colleague Dr. Bruce Klugherz, director of the Cardiac Catheterization Lab, both of AMS Cardiology and with Abington Hospital for 13 years, implanted the first device last month, but “lots more are scheduled in the near future,” Borge said. “I have a feeling it’s going to become a pretty popular thing,” he said. “It’s a pretty exciting new tool in the tool bag.”
Written By: Linda Finarelli
Source: Montgomery News
*Update: Download the Article PDF Here

